
Understand the members of the lubricant antioxidant family and their antioxidant mechanism in one article
What is a lubricant antioxidant?
Lubricant antioxidant is an additive that can effectively slow down the oxidation reaction of lubricants due to contact with oxygen during use, thereby extending the service life of lubricants and maintaining their stable performance. They prevent lubricants from deteriorating by terminating free radical reactions, decomposing peroxides or inhibiting metal catalysis, ensuring the normal operation of mechanical equipment and reducing maintenance costs.
Oxidation process of lubricants
The oxidation of lubricants begins due to the effects of light, heat, transition metals, etc., which produce free radicals. Free radicals react with oxygen to produce peroxy radicals, which react with other molecules to produce hydrogen peroxide and free radicals. Hydrogen peroxide further decomposes to produce alkoxy radicals and hydroxyl radicals. The result of the chain reaction is the further generation of ketones, aldehydes, organic acids, etc., and finally a condensation reaction to produce sludge and paint film, while increasing the viscosity of the lubricant.
No matter what kind of oxidation product, it is generally harmful. Oil sludge adheres to the metal surface, which may cause adhesion or wear of moving parts, block filters and oil pipelines, and reduce the amount of circulating oil. The acid generated by oxidation will corrode the metal. Oxidation is also related to the increase in oil viscosity, and the oxidation product itself acts like a catalyst, further accelerating oxidation.
From the reaction process, it can be seen that there are two ways to control oxidation:
① Prevent the formation of peroxides, that is, once peroxides are formed, they are destroyed to terminate the continued development of the chain or the development of free radicals. Therefore, the direct way to prevent the formation of peroxides is to add additives that have a strong affinity for oxides-peroxide decomposers and chain terminators, that is, antioxidants.
② Study the catalytic effect of inhibiting metal oxidation on oil products, that is, adding metal deactivators or passivators.
Antioxidant mechanism of antioxidants
The role of antioxidants is to eliminate the free radicals that have just been generated, or to promote the decomposition of hydroperoxides to prevent the chain reaction from proceeding.
(1) Antioxidant mechanism of amine antioxidants
The hydrogen atom connected to the nitrogen atom in the structure of amine antioxidants is the most active. The oxidation rate of the product is reduced by the reaction of active hydrogen with free radicals. The antioxidant molecules that lose active hydrogen to form stable free radicals are then used to capture free radicals and terminate the oxidation reaction.
Hydrogen atom donor:
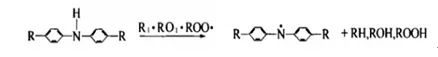
Chain termination:
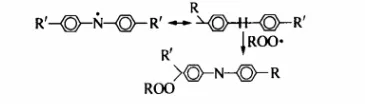
Or
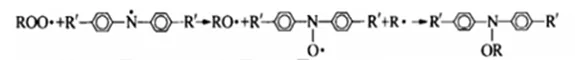
(2) Antioxidant mechanism of phenolic antioxidants
Phenolic antioxidants terminate the action of free radicals by giving a hydrogen atom or coupling reaction (i.e. termination reaction), thereby inhibiting or delaying the oxidation of the product.
Hydrogen atom donor:
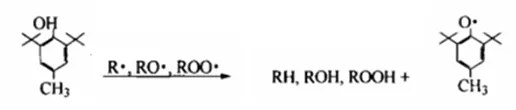
Chain termination:
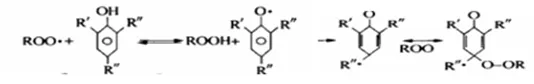
(3) Antioxidant mechanism of auxiliary antioxidants
For example, the sulfur atom in thioether is very chemically active. Under oxidation conditions, it is first attacked by electrophilic reagents to form sulfoxides, which are quickly oxidized to sulfones and then to ketones. The thioether group participates in the reaction of peroxides, causing the peroxides to decompose and inhibit the oxidation reaction.
Types of Antioxidants
Antioxidants can be divided into primary antioxidants (Class I antioxidants) and secondary antioxidants (Class II antioxidants). Antioxidants that can eliminate free radicals include aromatic amines, hindered phenols and their derivatives, which are called primary antioxidants; antioxidants that can decompose hydroperoxides include phosphorus- and sulfur-containing organic compounds, which are called secondary antioxidants.
(1) Amine Antioxidants
Amine antioxidants have significant antioxidant effects, especially outstanding high-temperature antioxidant properties. They also have discoloration properties, which can change the color of oil products. Lubricants with OSAIL® L060 and OSAIL® APAN added to UNPChemicals may darken in color during extended storage under light, but this does not affect their excellent antioxidant performance.
(2) Phenolic antioxidants
Hindered phenolic antioxidants are phenolic compounds with steric hindrance. They have significant anti-thermal oxidation effects and good non-discoloration and non-fouling properties. However, phenolic antioxidants with small molecular weight are generally more volatile and extractable at high temperatures, which affects the antioxidant performance. The application temperature is lower than that of amine antioxidants. UNPChemicals' OSAIL® T501 phenolic antioxidant has excellent high-temperature antioxidant performance due to its relatively high molecular weight. BHT antioxidants are widely used as antioxidants, stabilizers, and anti-gelling agents in general temperature oils due to their outstanding cost-effectiveness.
(3) Composite antioxidants
The combination of multiple types of antioxidants will have a synergistic antioxidant effect, and its antioxidant effect is often greater than the sum of the single use. Different types of antioxidants can terminate the chain reaction of oil oxidation at different stages, and their antioxidant effect is more prominent.
(4) Auxiliary antioxidants
Liquid high molecular weight phenolic antioxidants containing thioether groups, in addition to being the main antioxidants, can also be used as auxiliary antioxidants because the thioether groups they contain can decompose peroxides and have multifunctional activity. Phosphites can react with peroxides. As auxiliary antioxidants, they are often used in combination with phenolic antioxidants to have a synergistic effect.
Selection and use of antioxidants
The same antioxidant also shows different antioxidant effects in different oils. Generally, Class I and Class II base oils show good sensitivity to phenolic antioxidants, while Class III and Class IV base oils have good sensitivity to amine antioxidants.
The antioxidant mechanisms and effects of different types of antioxidants in the same oil are different. In applications, the synergistic effect of different types of antioxidants should be fully utilized and synergists should be used to obtain the best antioxidant effect.
Contact metals have a catalytic effect on the oxidation of lubricating oils, which is more obvious in the presence of water, because they accelerate the decomposition of peroxides to generate free radicals, trigger oil oxidation chain reactions, and accelerate oil oxidation. Trivalent or polyvalent transition metals (Co, Cu, Fe, Mn, Ni) with appropriate redox potentials have a strong effect in promoting oil oxidation. In the actual use of oil products, metallic copper is more common. In some cases, when antioxidants are used together with metal passivators (such as CSAIL® 3900), more prominent antioxidant effects are obtained.
During the use and storage of oil products, pay attention to factors that promote oxidation, such as light (ultraviolet rays), heat (high temperature), oxygen pressure, etc.
During the application, pay attention to the dissolution and dispersion of antioxidants in oil products. The degree of dissolution and dispersion will affect the antioxidant effect.
Metal deactivators are indispensable for all-round antioxidant systems
During the use of oil products, in the presence of oxygen, they are oxidized and deteriorated by heat and light. If the lubricating oil contains metals, such as copper, iron and other metal ions, even if the content is very low, they can also play a catalytic role, accelerate the free radical chain reaction in the oxidation process of oil products, accelerate the oxidation rate of oil products, and generate acid, sludge and precipitation.
Acids can cause corrosion and wear of metal parts, while sludge and precipitation can make oil thicker, causing the piston ring to stick and the oil circuit to be blocked, which in turn reduces the performance of the oil. Therefore, it is very important for lubricants to prevent metal ions from promoting oxidation.
Metal passivators, also known as metal passivators or corrosion inhibitors, are lubricant additives that inhibit the catalytic effect of metals on oxidation and corrosion. For lubricants, antioxidants and metal deactivators are usually used in combination to exert the synergistic effect of the two, thereby greatly improving the antioxidant capacity of the oil.
The binary compound of phenolic and amine antioxidants is better than the use of one antioxidant alone, while the ternary compound of phenolic, amine antioxidants and metal deactivators can play a better synergistic role.
Professional Lubricant Additive Manufacturer
UNPChemicals, aka Luoyang Pacific United Petrochemical Co., Ltd., focuses on the application and development of special lubricating grease additives such as MoDTC, MoDTP, molybdenum amide, thiadiazole metal deactivators, and phosphate esters. With nearly 30 products in seven series, including extreme pressure anti-wear agents and special grease additives, it is a global manufacturer of special lubricating grease additives and a national high-tech enterprise with great influence and leading role in the industry.