
Chelating effect of metal deactivators
What is metal deactivator?
Metal deactivator, also known as metal passivator, is an additive used to prevent metals from promoting the auto-oxidation of fuel oil, lubricating oil and other oil products. Among various metals, copper has the greatest promoting effect on the auto-oxidation of gasoline and aviation jet fuel, followed by iron and aluminum. When chromium and copper coexist, the promoting effect is more significant. The mechanism of action of metal deactivators is mainly to form an inert barrier film that prevents catalytic oxidation and/or anode and/or cathode reactions that cause corrosion, and slows down the oxidation rate of oil products by inhibiting the catalytic effect of metal ions, thereby extending the service life of oil products. Common types of metal deactivators include benzotriazole derivatives, thiadiazole derivatives, nitrogen-containing compounds and heterocyclic compounds. These additives are usually used in conjunction with antioxidants and anti-gelling agents to complement each other, effectively preventing oil deterioration, reducing the formation of acid, sludge and precipitation, and avoiding corrosion, wear of metal parts and oil line blockage.
Classification and characteristics of metal deactivators
1. Benzotriazole derivatives
Features: Benzotriazole derivative metal deactivators have good antioxidant properties and the ability to inhibit copper corrosion. They can form stable chelates with metal ions, thereby inhibiting the catalytic effect of metals on oil oxidation. For example, CSAIL® T551 metal deactivator is a typical benzotriazole derivative with excellent antioxidant properties and copper corrosion inhibition. This type of additive is usually used to improve the oxidation stability of oil products, and has a significant synergistic effect when used in combination with antioxidants
2. Thiadiazole derivatives
Features: Thiadiazole derivative metal deactivators have excellent anti-wear, anti-oxidation and anti-corrosion properties. They can form a protective film on the metal surface to effectively prevent the catalytic effect of metal ions. For example, CSAIL® T561 metal deactivator is a thiadiazole derivative with good antioxidant, anti-corrosion and anti-wear properties, and is widely used in oil products. This type of additive can significantly improve the oxidation stability of oil products and play a good role in resisting copper flake corrosion in anti-wear hydraulic oils.
3. Multifunctional metal deactivator
Features: Multifunctional metal deactivator not only has metal deactivation performance, but also has other functions, such as extreme pressure anti-wear, thermal oxidation stability and rust resistance. For example, T581 multifunctional metal deactivator combines extreme pressure and metal deactivation chemical groups in one, which can solve the contradiction of using sulfur-containing extreme pressure agents and metal deactivators in lubricant formulations at the same time. The application of this type of additive in lubricating oil can improve the overall performance of lubricating oil, and is widely used in various oil products such as turbine oil, oil film bearing oil, industrial gear oil, transformer oil and circulating oil.
4. Amines
Features: Amine metal deactivators such as zinc dialkyl dithiophosphate (ZDDP) are widely used in engine oils and can effectively neutralize the catalytic effect of metals. This type of additive inhibits the catalytic effect of metals on oil oxidation by forming stable complexes with metal ions.
5. Other types
Features: It also includes some sulfide and other organic compound metal deactivators, which inhibit the catalytic effect of metals through different chemical mechanisms.
Chelating effect of metal deactivators
Today, let's talk about additives similar to hairy crabs. Crabs have two large claws, called "ao". These two large claws are like two big hands that can clamp what they want to catch. The way to play with crabs is as follows:
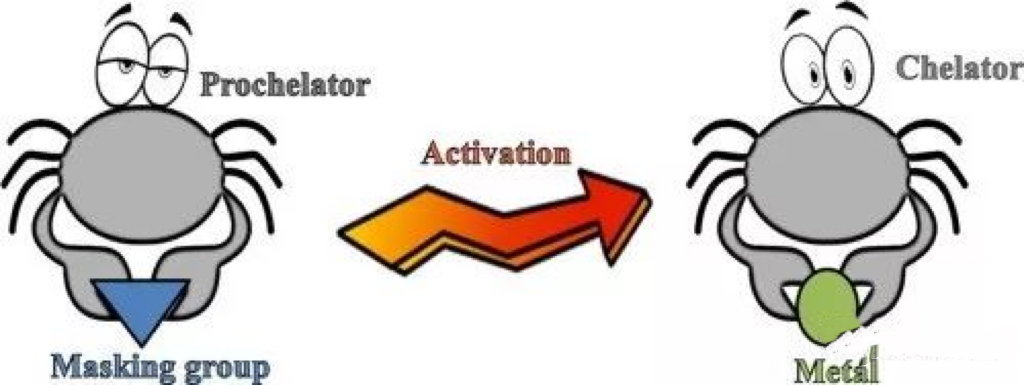
This picture basically tells you what chelation is. The main body that is clamped, now it seems, mainly refers to metal ions.
So why do we need to clamp these metal ions? The reason is very simple, that is, we don't want these metal ions to run outside. These ions have a very wide range in a broad sense. It can be said that for most ions, we can find chelating agents to chelate them, it's just a matter of whether we need them or not.
In our lubricating oil industry, chelating agents are not mainstream additives, because we actually have very few main cations that we need to clamp. The most important cation is the copper ion that we are all familiar with, and the other is calcium and magnesium ions.
The main use of chelating agents is in the metal cleaning and daily chemical cleaning industries, and the cations to be chelated include iron, calcium, magnesium, copper, sodium, etc.
To explain the principle of chelate, the official explanation is as follows:
The complex ion formed by a simple positive ion (called the central ion) and several neutral molecules or ions (called ligands) is called a complex ion (also called a complex ion), and the compound containing the complex ion is called a coordination compound. The central ion of the complex is combined with the ligand through a coordination bond. If there is only one coordinating atom in the ligand, only one coordination bond can be formed between the central ion and the ligand. Some ligand molecules contain more than two coordinating atoms. When there are two to three other non-coordinating atoms between the two atoms: this ligand can form more than two coordination bonds with the central ion (or atom) at the same time, and form a special structure including two coordinated five-membered or six-membered rings. This complex is called a chelate. Chelates are more stable than general complexes.
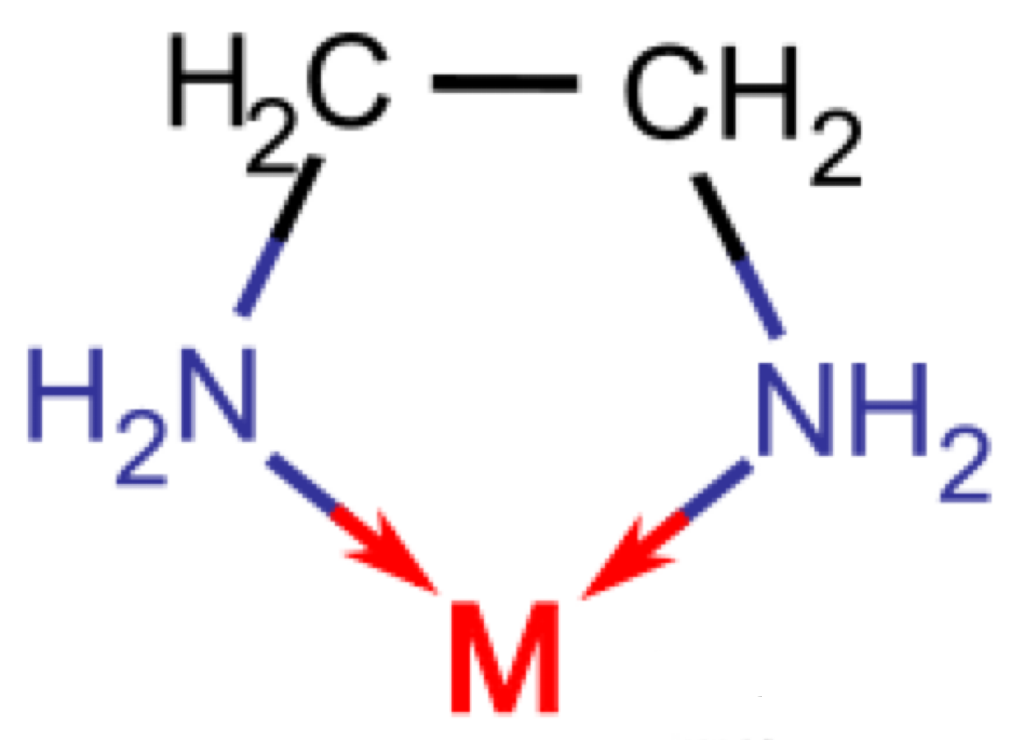
To explain in simple terms, the positive ion is the cation that needs to be clamped. The crab's hand is called a ligand. When there is only one ligand, it cannot clamp tightly. Multiple clamps are better, so that the cation can be grasped tightly. Imagine that if there is only a single ligand, it is like we only use chopsticks to clamp things, which is not firm (as shown above). At this time, if a special structure of five or six rings can be formed, it will be quite stable, just like forming a crab shell. It takes a lot of strength to break free. There is no point in saying more. Let's take a look at the following example: T1201, its structure is as follows:
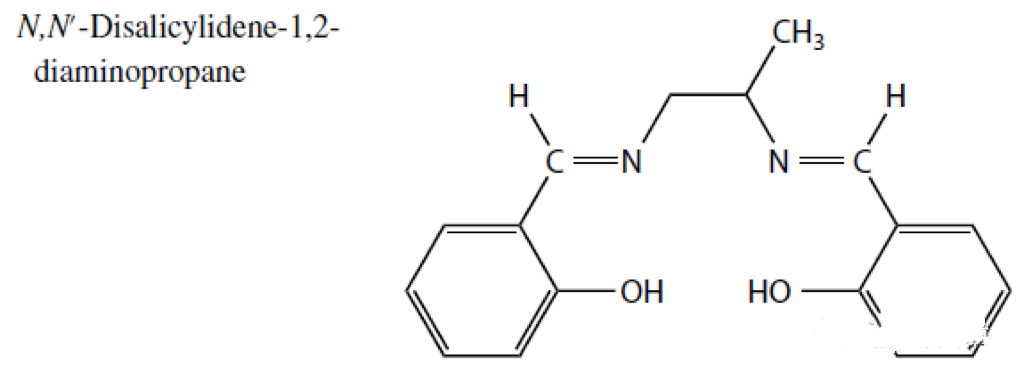
Then he likes the metal copper element very much, and the two big claws like to catch copper, which becomes the following picture:
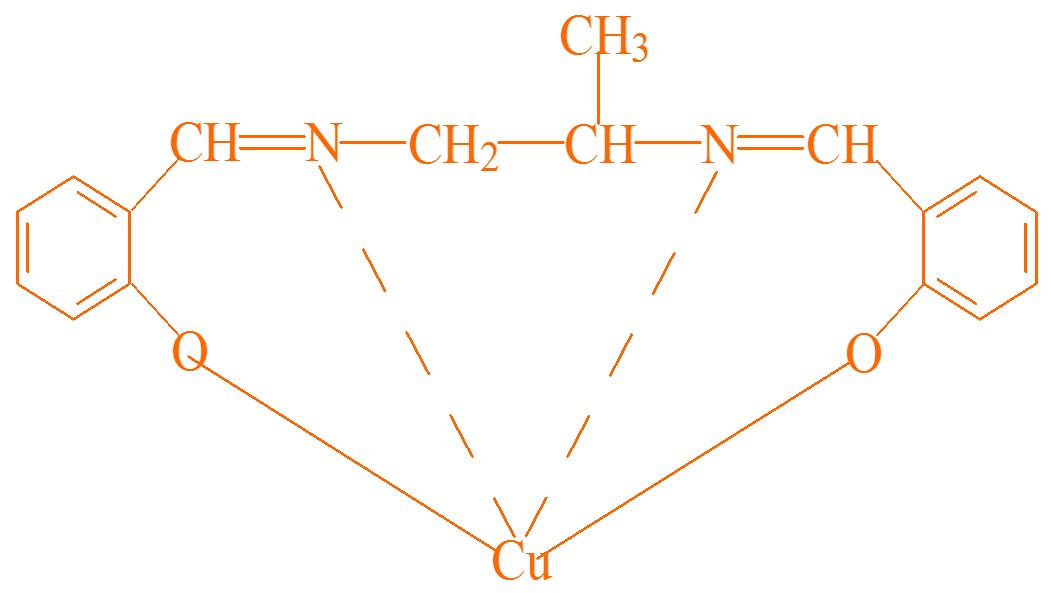
How about it, like a big crab? This kind of state is generally called a chelator. The method is to catch the copper ions, so that the copper ions will not run out. If they do not run out, they will not come into contact with bad people, nor can they harm good people. Therefore, as the victim, this thing is a corrosion inhibitor (which solves the problem of metal being corroded too quickly), and as the attacker, this thing is a metal passivator, which is used to prevent metal from going wild outside. For example, copper can go out as a catalyst for lubricant oxidation, or combine with other film-forming substances. The biggest feature of chelation is that a layer of complex is formed on the metal surface. This complex is a chemical change, so chelation reactions are typical chemical reaction films, and many of the anti-corrosion adsorption films mentioned above are physical films.
But for T1201, its main application industry is not our lubricant field, but the fuel oil field.
Next, let's talk about the two major protectors of metal passivators related to our industry, represented by CSAIL® T561 and CSAIL® T551:
CSAIL® T561
CSAIL® T561 is thiadiazole and its derivatives. Thiadiazole is as shown in the figure below:
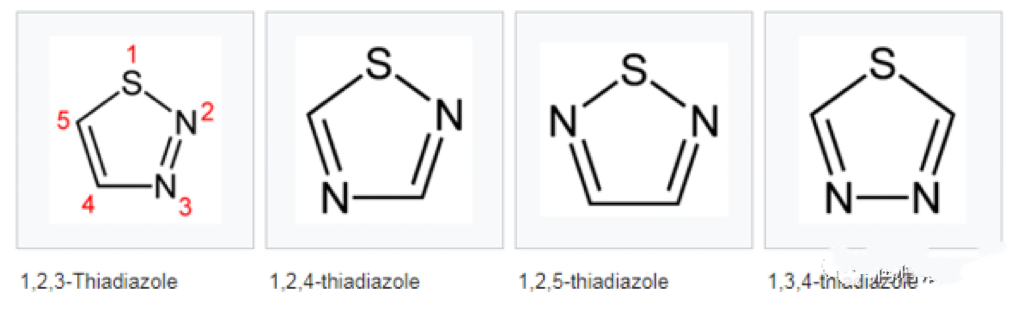
But thiadiazole is a basic model, belonging to the lowest configuration. The commonly used intermediate should be the following one, called DMTD. DMTD is actually a hot topic at the moment, because it can not only be made into a single agent, but also can be connected to many other substances.
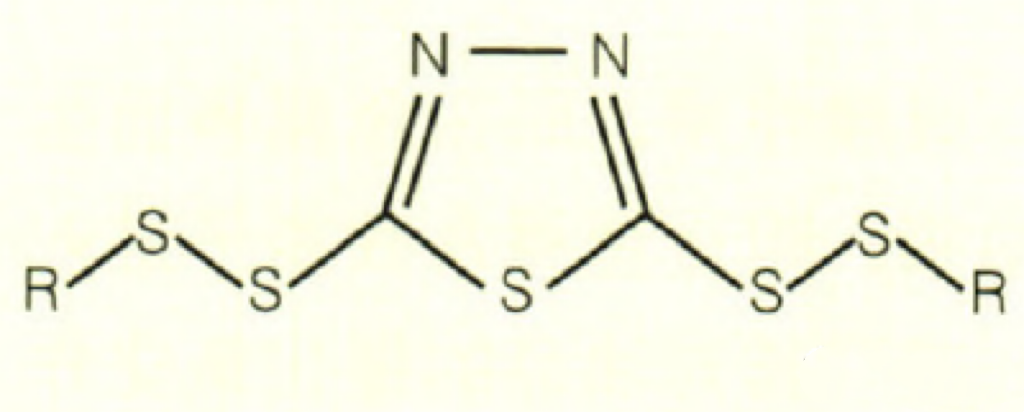
Then there is CSAIL® T561. CSAIL® T561 is actually a polysulfide of thiadiazole. Its biggest feature is that there is more sulfur, and the benefit of more sulfur is that the antioxidant performance is improved, the extreme pressure performance is improved, and different R groups can achieve different functions, which is why it is getting attention.
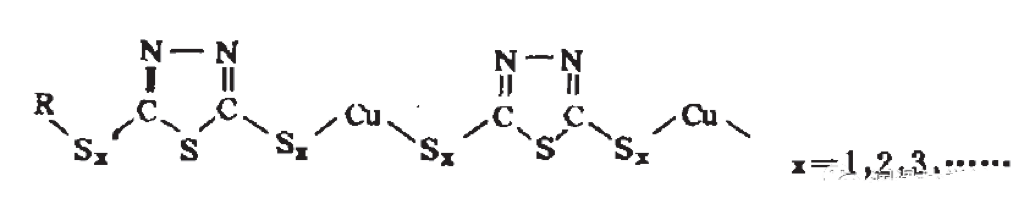
The biggest feature of thiadiazoles is that they have two active points, sulfur and nitrogen. Strictly speaking, thiadiazoles can also chelate copper, but in fact, due to the presence of sulfur, especially the derivatives of thiadiazoles are mainly derived from the carbon atoms in thiadiazoles, and more sulfur is added. In this way, thiadiazoles and their derivatives as passivators are not just as simple as chelation, but react with free metals to form films.More sulfur elements can capture copper elements, and can also capture free radicals with the presence of oxygen. In this way, although it is a passivator, it is already in the range of antioxidants. In practice, it is also used in this way. Even in the classification of additives in my country, metal passivators are classified in the number of antioxidants. After the introduction of sulfur elements, it is not just a chelation, but there will be a direct reaction called a metal passivation film. The existence of this film, as we have always mentioned, has certain intersections and conflicts with anti-corrosion films, anti-wear agent films, and extreme pressure films. From the research conclusions of many scholars, passivation films are the mainstream of passivators.
Application of CSAIL® T561: In the alkaline high-zinc anti-wear hydraulic oil formula, adding 0.03-0.05% CSAIL® T561 metal deactivator, the oil can pass the Denison HF-2 hydrolysis stability test. In the internal combustion engine oil formula, adding 0.4-0.5% T561 metal deactivator can increase the TFOUT induction period of the oil by more than 60 minutes. In addition, CSAIL® T561 can also be used in industrial gear oil, turbine oil, gasoline engine oil, emulsified oil and other oil products.
CSAIL® T551
The last one is BTZ, which is benzotriazole and its derivative system, such as CSAIL® T551. The principle of its passivation film is as follows:
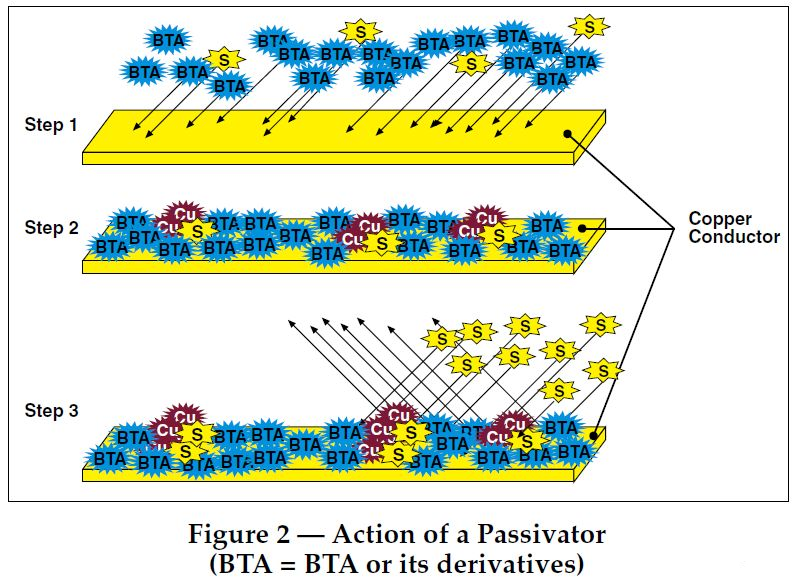
Benzotriazole derivatives such as CSAIL® T551 can form stable chelates with metal ions (such as copper ions). This chelation is mainly achieved by the coordination of nitrogen atoms in the benzotriazole molecule with metal ions. Specifically, the nitrogen atom in the benzotriazole molecule provides lone pairs of electrons to form coordination bonds with metal ions, thereby reducing the activity of metal ions and inhibiting the catalytic effect of metals on oil oxidation. For example, CSAIL® T551 can react with copper ions to form stable chelates, a process that provides experimental evidence for existing theories.
Application of CSAIL® T551: CSAIL® T551 is widely used in a variety of lubricating oils, such as turbine oil, transformer oil, gear oil, general machine tool oil, etc. The general dosage is 0.03% to 0.05%, and at the same time it can reduce the dosage of the main oxidant by 30% to 40%. For example, in transformer oil, CSAIL® T551 can significantly inhibit copper corrosion and extend the service life of transformer oil.